Medtronic InterStim® Exemplary Care and Experience

Aguirre Specialty Care is extremely proud to announce that Dr. Oscar A. Aguirre’s Medtronic InterStim program has existed for 20 years – since 2001. It was then that Dr. Aguirre was trained by the inventor of InterStim Therapy, Dr. Richard Schmidt, who was professor of urology at the University of Colorado. Dr. Aguirre became the first surgeon in private practice to offer InterStim Therapy to his patients and thus is the second most tenured implanter in Colorado. Dr. Aguirre has consistently remained one of the top implanters in the state of Colorado, averaging 40 implants per year.
Dr. Aguirre is recognized, by patients and colleagues alike, as a physician who demonstrates exemplary use of the InterStim System and who is committed to patient care for those suffering from symptoms associated with overactive bladder, fecal incontinence and non-obstructive urinary retention.
InterStim® – Treatment for Over-active Bladder, Fecal Incontinence and Urinary Retention
Bladder control problems affect millions of Americans. If you’re one of them, you know how these conditions, like over-active bladder (OAB), fecal incontinence (involuntary loss of stool) and urinary retention can interrupt your life.
You may have tried changing your diet, doing kegal exercises and physical therapy, or medications with unpleasant side effects, but the results just aren’t what you hoped.
Communication between your brain and bladder is critical. That’s why conventional treatments may not produce the results you want — they don’t target the miscommunication between your bladder and brain.
Unlike conventional treatments, Medtronic Bladder and Bowel Control Therapies delivered by the InterStim system use gentle nerve stimulation to correct the bladder/bowel-brain communication pathway and restore bladder and bowel function.
Medtronic Bladder and Bowel Control Therapy delivered by the InterStim system restore bladder and bowel function by gently stimulating the sacral nerves. It’s sometimes called sacral neuromodulation (SNM). With this therapy, you may experience fewer trips to the bathroom, fewer accidents and more confidence as you get back to the activities you enjoy.
InterStim is safe, FDA-approved and minimally invasive. It’s been helping people improve their quality of life for more than 20 years.
- 84% satisfaction among those who use it
- 3x greater improvements in quality of life compared to medications
- 82% of people maintain success at five years with InterStim therapy
- 45% of people achieve complete continence at five years with InterStim therapy
Unlike other bladder and bowel control therapies, sacral nerve stimulation with InterStim Therapy (or Axonics Therapy) is the only therapy that allows you to try it first. It’s called an evaluation or trial — like a test run, not a long-term commitment.
Here’s how it works
- The simple evaluation starts at our outpatient facility
- A lead (thin wire) is placed in your upper buttock along the nerves that control your bladder
- The lead attaches to a small external device worn discreetly under your clothes
- Stop, start or adjust the therapy with a Samsung controller
- Go about most of your normal activities for three to six days
- Track your symptoms in a diary to see if they improve
After the end of your trial, talk with our providers about the results. Did it feel successful? Did you see symptom improvement? Together, we will decide if the long-term therapy is the right choice. If it is, we can replace the external device with an implantable device called a neurostimulator during a short, out-patient procedure.
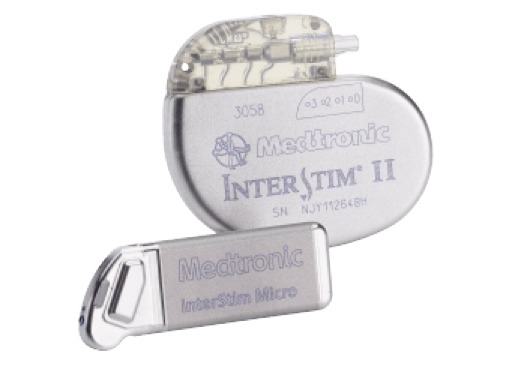
What is InterStim™ II?
The recharge-free InterStim II system gives patients freedom from a recharging routine, the hassle of recharging components and a reminder they have a disease. InterStim II is simple, convenient and low-maintenance. InterStim II now allows full-body 1.5 and 3 Tesla MRI conditional scans with SureScan™ MRI technology.
What is InterStim™ Micro?
The U.S. Food and Drug Administration recently approved the InterStim™ Micro neurostimulator, the market’s smallest and fastest rechargeable device to deliver sacral neuromodulation (SNM) therapy. It offers a smaller size compared to InterStim II and a longer battery life. InterStim Micro also allows full-body 1.5 and 3 Tesla MRI conditional scans with SureScan MRI technology.
What are the benefits of the new InterStim™ systems for patients with OAB or FI?
Medtronic is the only company to offer patients the freedom to choose between a rechargeable or recharge-free sacral neuromodulation device to best match their preferences, lifestyle and treatment goals. Both InterStim Micro and InterStim II are full-body MRI conditional and deliver the same therapy and long-term relief.
The recharge-free InterStim II system is simple and convenient with lower maintenance and fewer time commitments. This specific system gives patients the freedom from a recharging routine, the hassle of recharging components and a reminder they have a disease.
The InterStim Micro rechargeable system is the smallest device on the market with the fastest rechargeability and is stronger than other manufacturers’ batteries. It features proprietary Overdrive™ battery technology — a battery with virtually no loss in capacity over time. The new battery technology allows patients to choose how and when they want to charge their device — from as often as once a week or as infrequent as once per month, depending on the patient’s preference and device settings. There is no battery fade at 15 years* and patients can restart their therapy after extended breaks in time.
To help decide which system (recharge-free vs. rechargeable) best fits your needs and lifestyle, please speak with our providers and watch the video below.
How does InterStim Micro compare in size to other neurostimulators?
The InterStim Micro neurostimulator is about half the size of the other rechargeable device on the market, Axonics Therapy.
The most common adverse events include pain at implant sites, new pain, lead migration, infection, technical or device problems, adverse change in bowel or voiding function, and undesirable stimulation or sensations. Any of these may require additional surgery or cause return of symptoms.
Visit Medtronic.com to learn more.
FAQs
Q: What is Medtronic Bladder and Bowel Control Therapy delivered by the InterStim system?
A: This therapy targets the nerves that control your bladder and bowel to help it function normally again.
Q: How does it work?
A: Medtronic Bladder and Bowel Control Therapy delivered by the InterStim system restores bladder and bowel function by gently stimulating the sacral nerves.
Q: Why does it treat the sacral nerves?
A: It’s thought that bladder and bowel control problems are caused by miscommunication between the brain and the sacral nerves, which control the bladder and bowel and muscles involved in urination.
Q: What are the benefits of this therapy?
A: With this therapy, you may experience fewer trips to the bathroom, fewer accidents and more confidence as you get back to the activities you enjoy.
Q: What are the potential side effects or complications?
A: Implanting an InterStim system has risks similar to any surgical procedure, including swelling, bruising, bleeding and infection. Talk with your doctor about ways to minimize these risks.
Q: What makes this therapy different than other options?
A: It is the only advanced therapy for OAB that is clinically superior to oral medication. It’s also the only therapy that lets you try it first during an evaluation.
Q: How long does the relief last?
A: This therapy significantly reduced symptoms of OAB and non-obstructive urinary retention in people treated for five years. Your experience may be different.
Q: Will this therapy cure my condition?
A: No. It can be effective, but it’s not a cure. If the neurostimulator is turned off or removed, symptoms can return.
Q: What does the stimulation feel like?
A: Most people describe it as a slight pulling, tingling or fluttering sensation in the pelvic area. It should not be painful. Stimulation settings can be adjusted and sensations will vary from person to person.
Q: Can I get an MRI when I receive this therapy?
A: Getting a full-body MRI scan may be possible under specific conditions. For more information, talk with your doctor.
Q: Will insurance cover the costs?
A: Medicare and most private insurance companies cover this therapy. Talk with our business office to learn more about your insurance coverage.
Q: How long has this therapy been around?
A: It is an FDA-approved therapy doctors have used to treat bladder and bowel control problems for nearly 20 years. More than 250,000 people have received this therapy.
Q: What do patients say about their experience with InterStim Therapy?
A: See reviews from patients




